METHODS OF TRIFLUOROMETHYL CARBENE C-H INSERTION

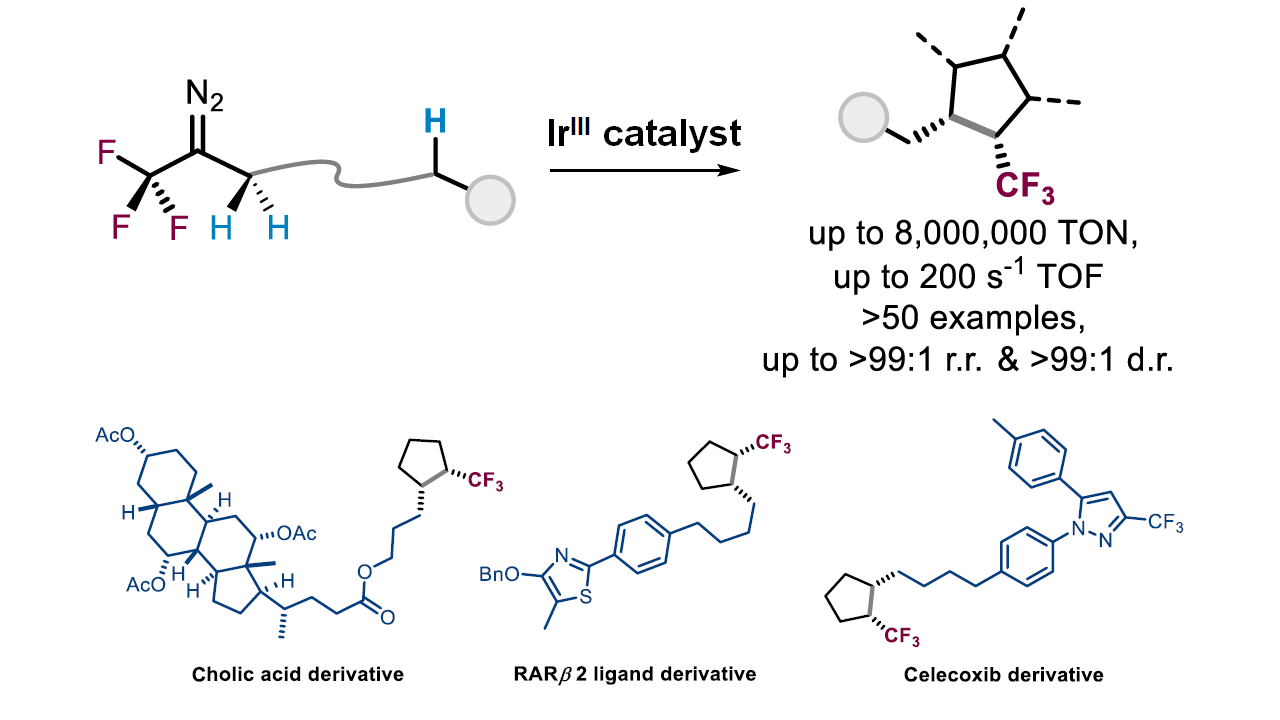
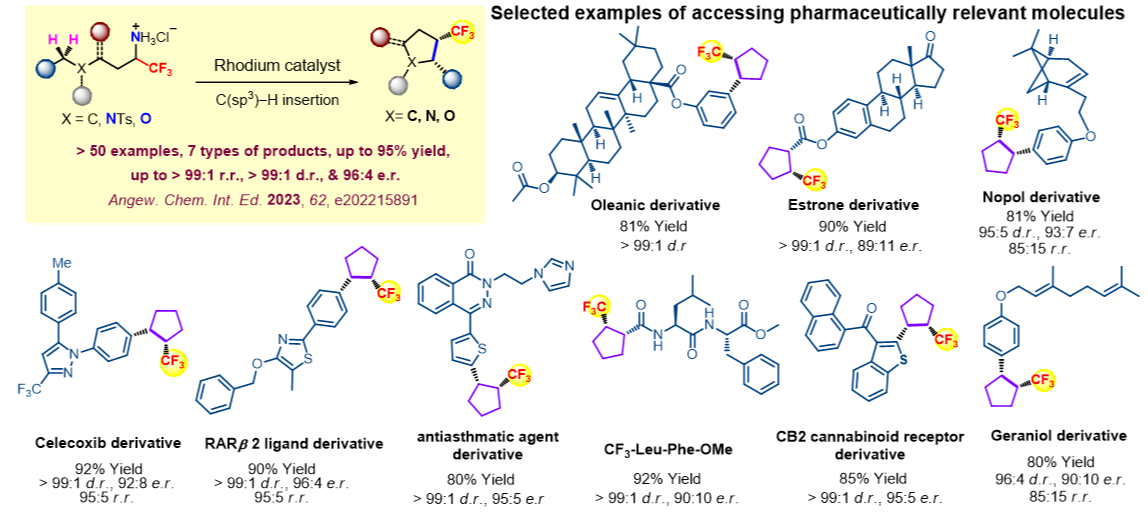
Ir-catalyzed intramolecular α-CF3-α-alkyl carbene aliphatic C–H insertion to afford an array of di-, tri-, and tetra-substituted CF3-bearing cyclopentanes in a highly site- and diastereo-selective manner. An unprecedentedly high TON (up to 8,200,000) has been reached for accessing tri-substituted CF3-bearing cyclopentanes. Further application to the synthesis of tri-substituted CF3-bearing cyclopentane analogues of natural products, pharmaceuticals, sugars and peptides is demonstrated.
TONs of catalytic functionalization of robust C–H bonds, especially for unactivated C(sp3)–H bonds, are typically <10,000 in the literatures. Therefore, improving turnover number of unactivated C–H bond functionalization to the same practical level as hydrogenation and cross-coupling is daunting.
- An unprecedentedly high TON (up to 8,200,000) has been reached for accessing tri-substituted CF3-bearing cyclopentanes by means of C-H activation
- Developing a new approach to afford an array of di-, tri-, and tetra-substituted CF3-bearing cyclopentanes in a highly site- and diastereo-selective manner
- Identifying the importance of iridium catalyst for high product turnover number
- Multi-substituted products with high site- and diastereo-selectivity and yield
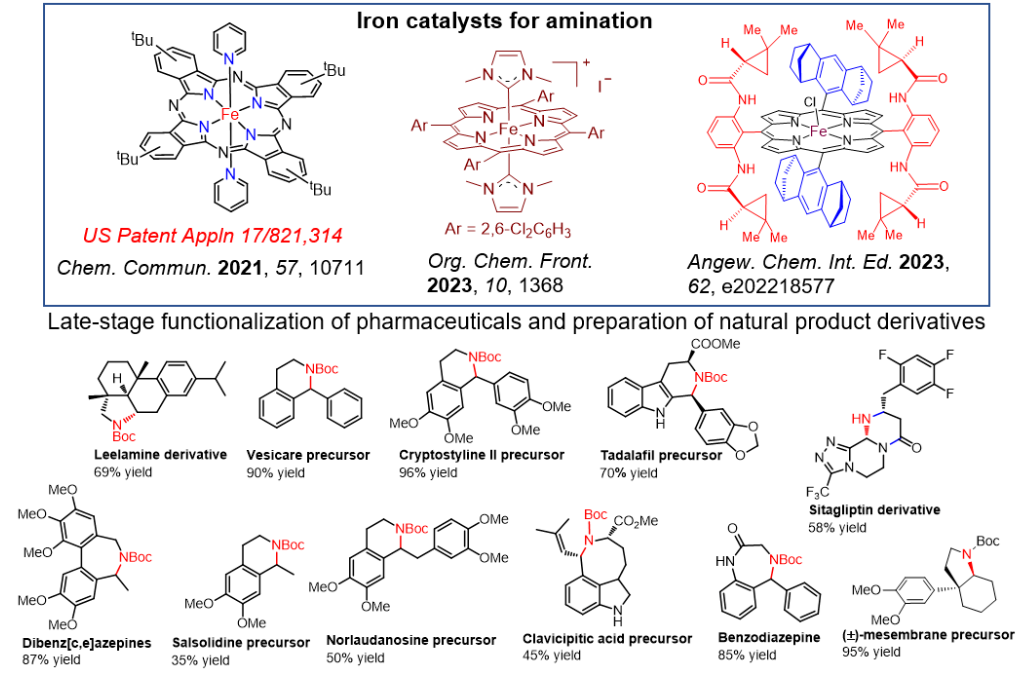
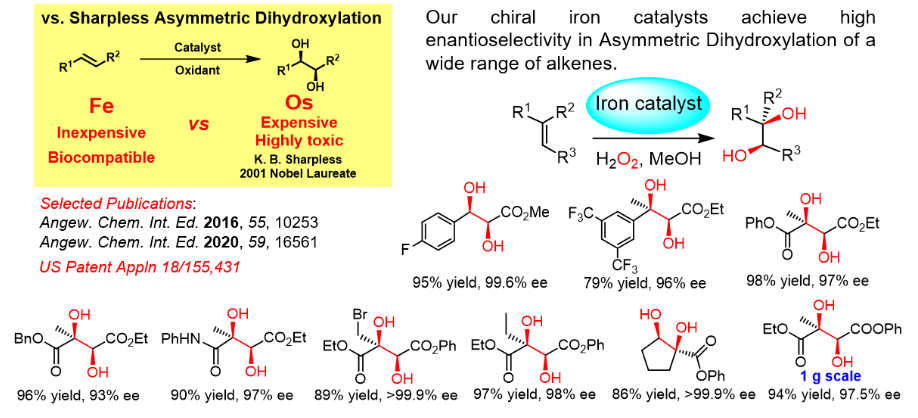
- for synthesis of potentially pharmaceutically active molecules containing trifluoromethyl-bearing five-membered ring structures
Laboratory for Synthetic Chemistry and Chemical Biology (LSCCB) is an R&D centre funded by Health@InnoHK progamme of Innovation and Technology Commission of HKSAR. Established in 2020, LSCCB is operated with tripartite joint collaboration of The University of Hong Kong, Imperial College London and Peking University. LSCCB aims at integrating chemical and biomedical sciences to develop new molecular medicines and diagnostic tools for the treatment and analysis of human diseases, in particular, cancer. LSCCB currently assembles more than 20 principal investigators for 4 major research programmes including (a) Synthetic Chemistry; (b) Chemical Biology of Natural Products and Chinese Medicine; (c) Metal Anticancer Medicine, Diagnostics and Theranostics; and (d) Multi-Omics and Innovative Analytical Technologies for InnoHealth.