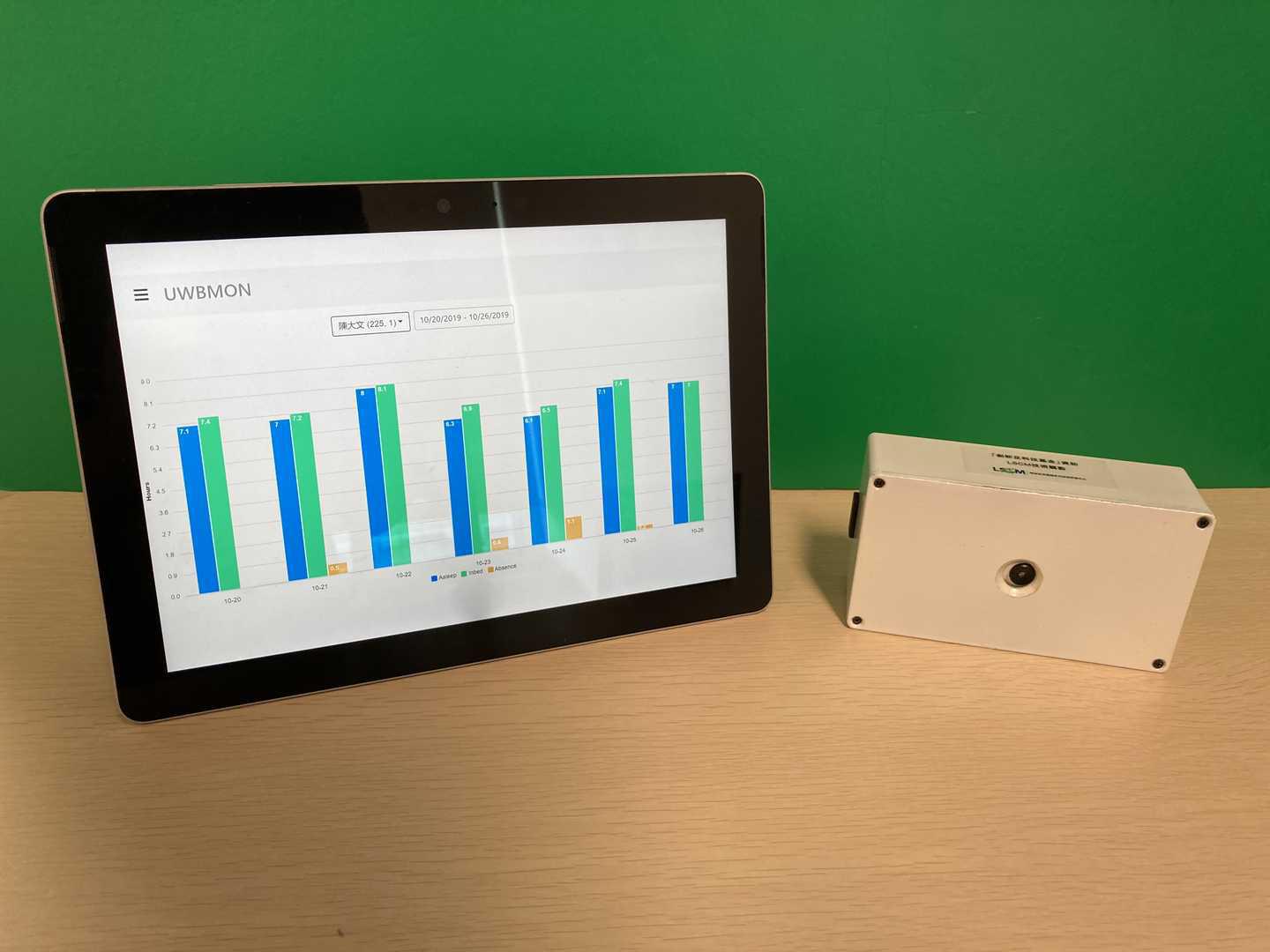
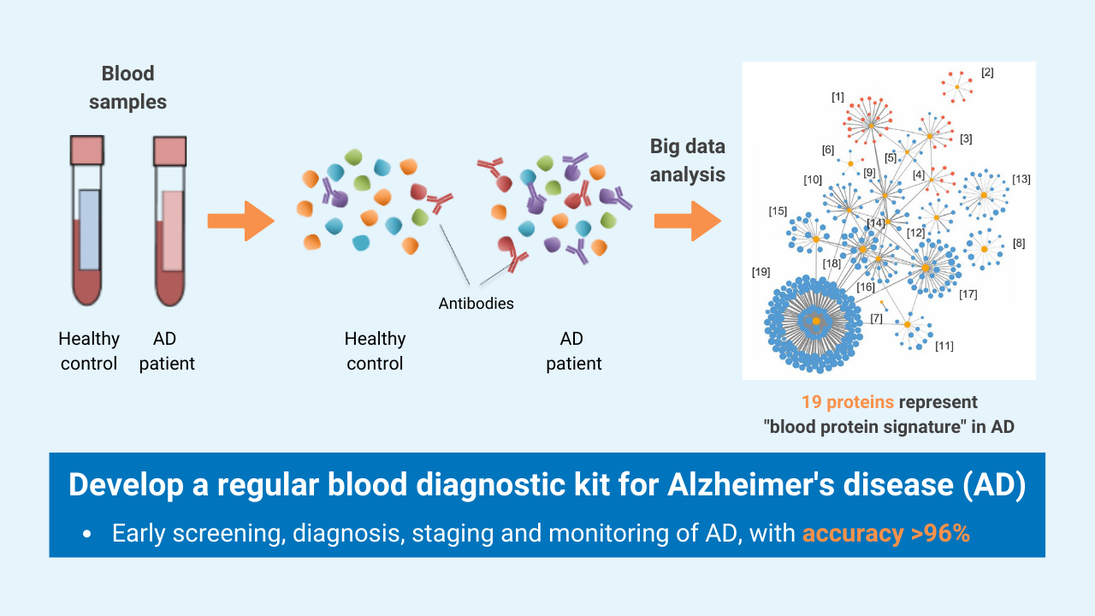
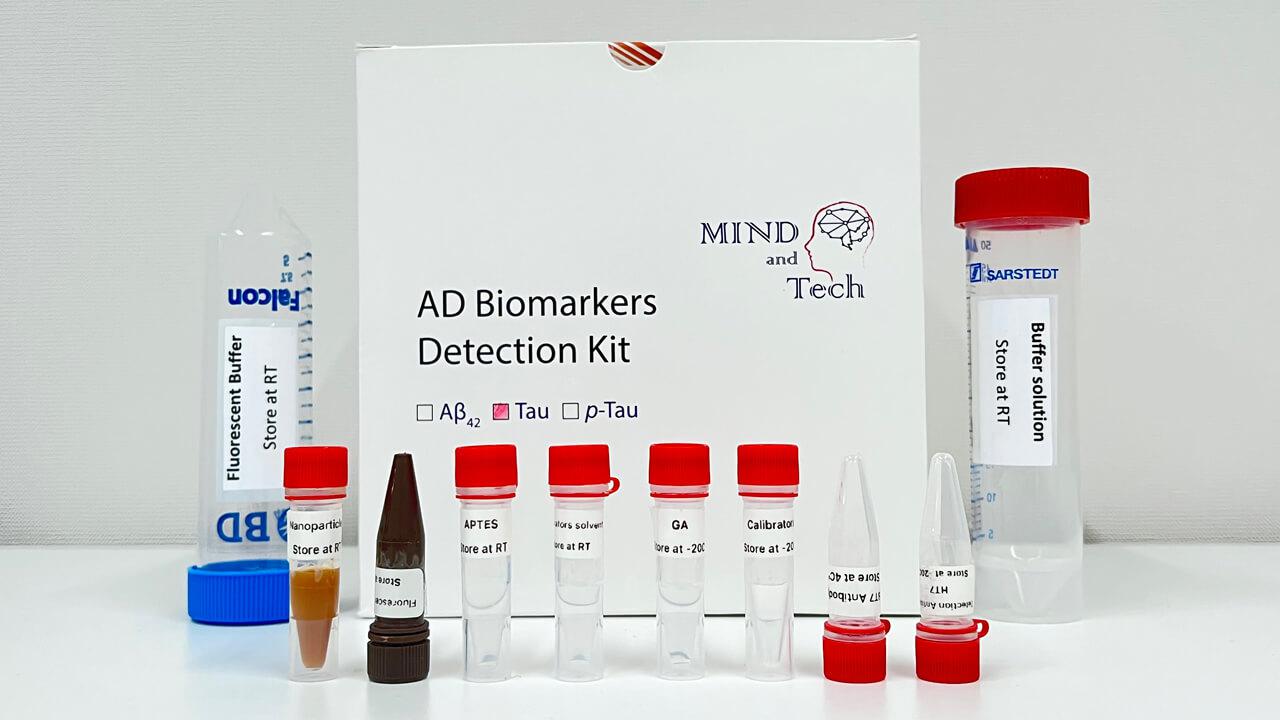
Blood Test for Early Detection and Personalized Management of Alzheimer’s Disease

The groundbreaking multi-protein blood test offers a simple and robust solution for the early detection and monitoring of Alzheimer’s disease (AD) and mild cognitive impairment (MCI). By evaluating multiple AD-associated biological processes, the test provides a comprehensive analysis of an individual’s disease status, facilitating patient stratification and the development of personalized treatment.
Alzheimer’s disease (AD) affects over 50 million people worldwide, imposing significant economic and societal burdens. In 2023 and 2024, two anti-amyloid drugs were approved as the first disease-modifying treatments, targeting MCI and mild AD-related dementia with elevated amyloid beta (Aβ) levels in the brain. However, many individuals with these conditions remain undiagnosed and untreated, primarily due to challenges in early detection. This novel blood test provides a highly accurate, non-invasive, and cost-effective solution for identifying individuals with MCI and mild AD while simultaneously detecting elevated Aβ levels, addressing this unmet medical need.
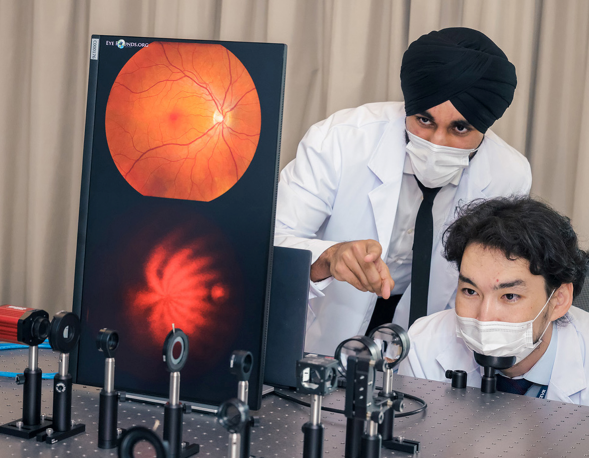
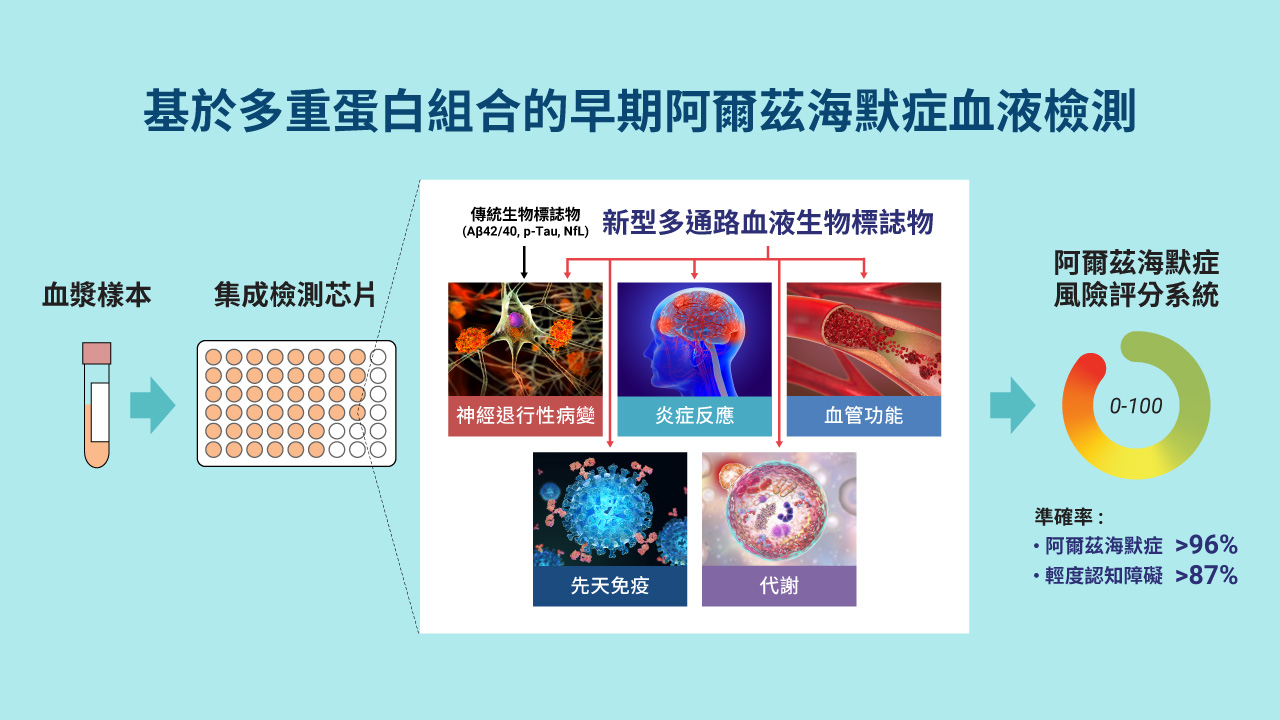
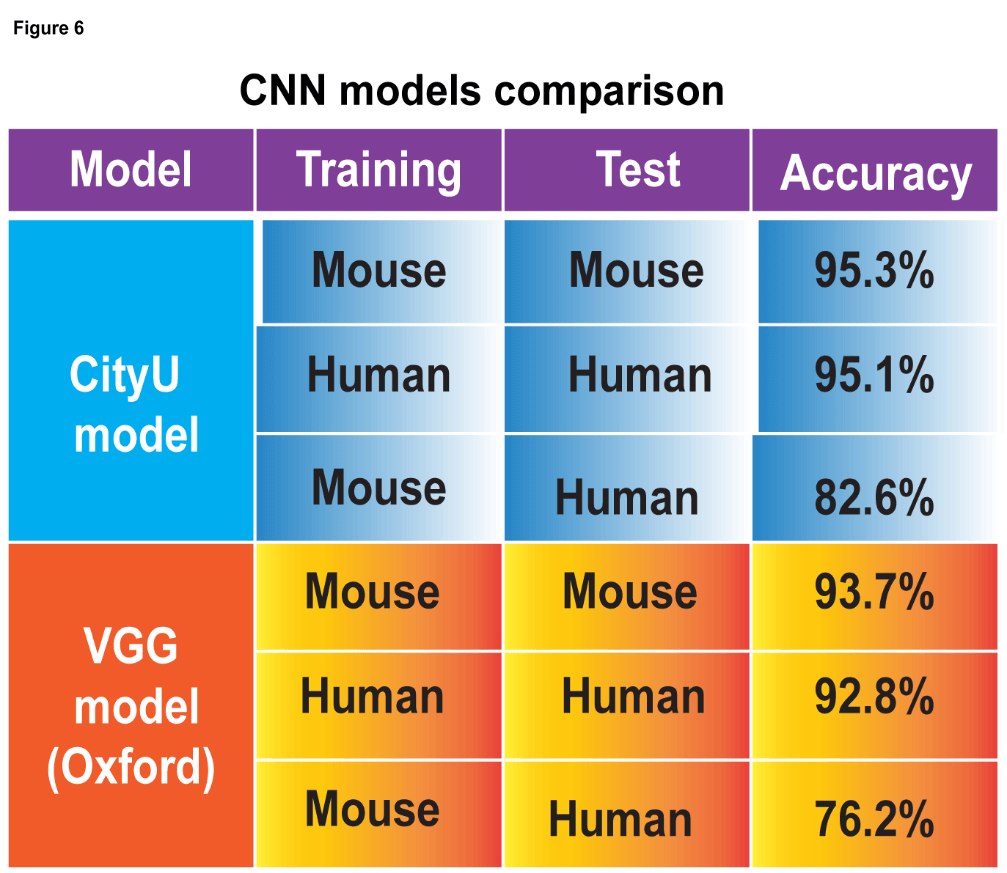
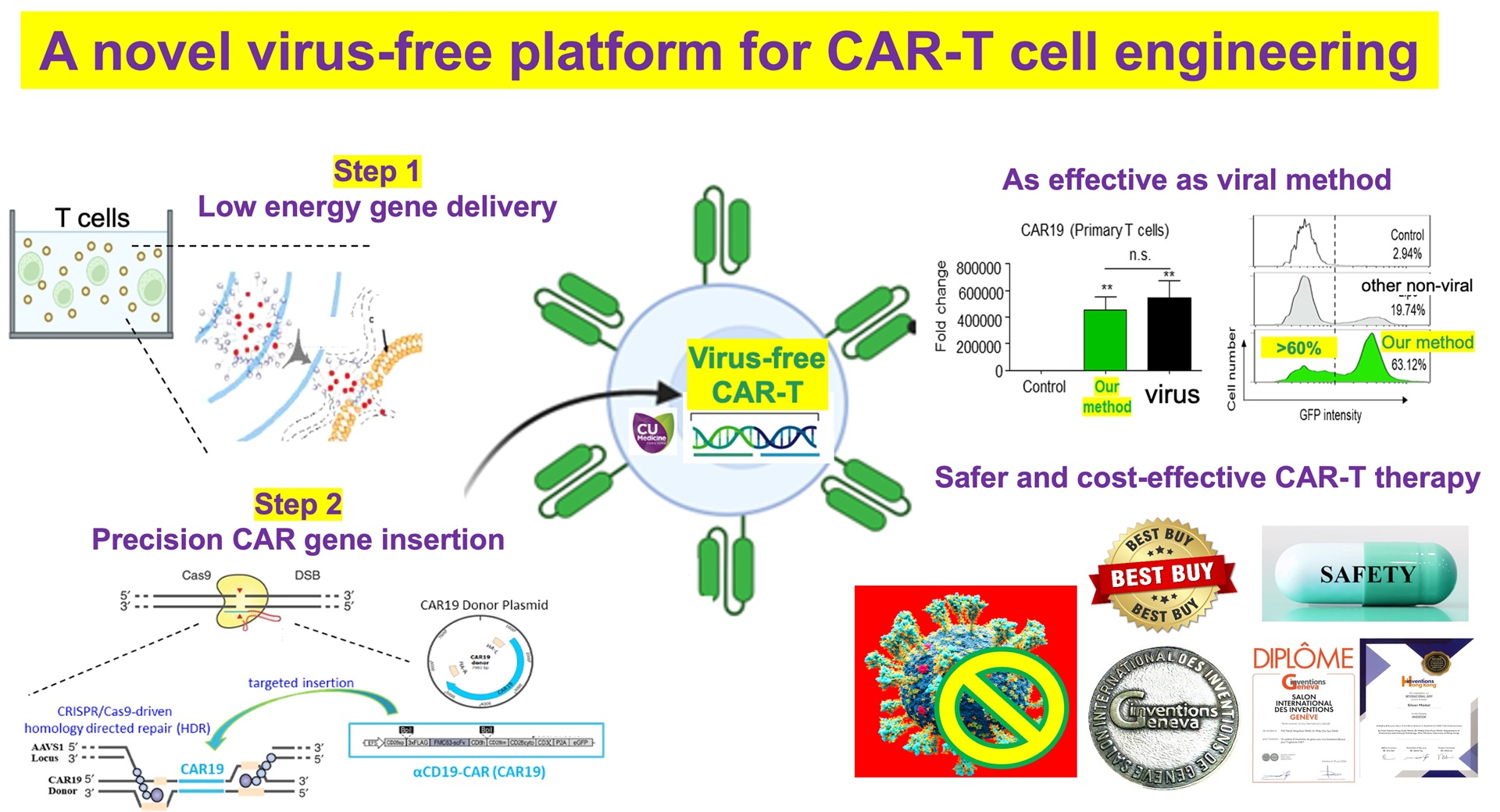
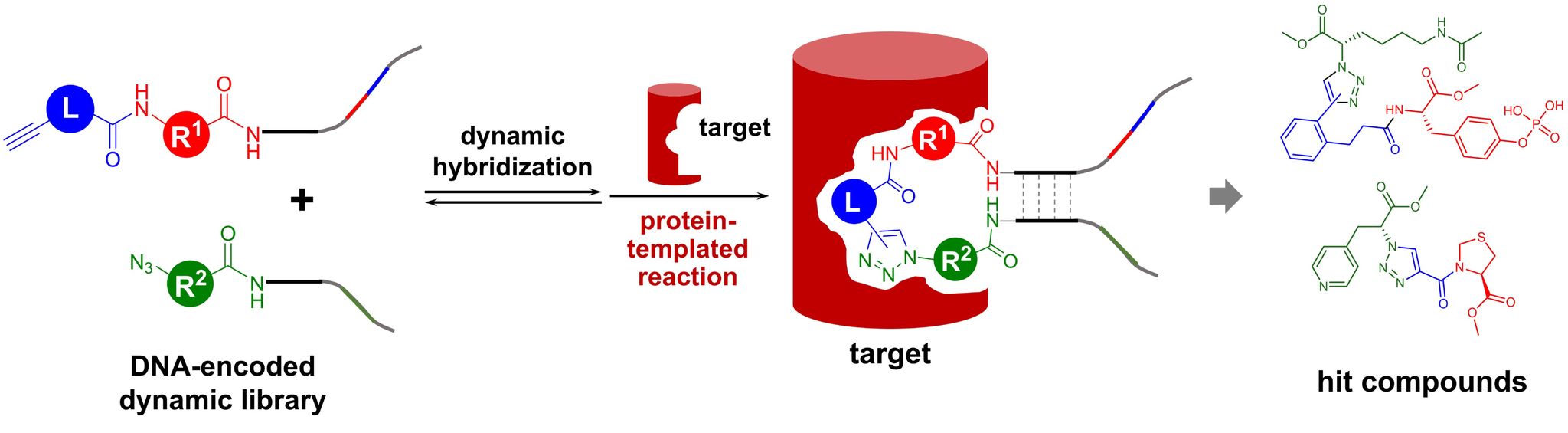
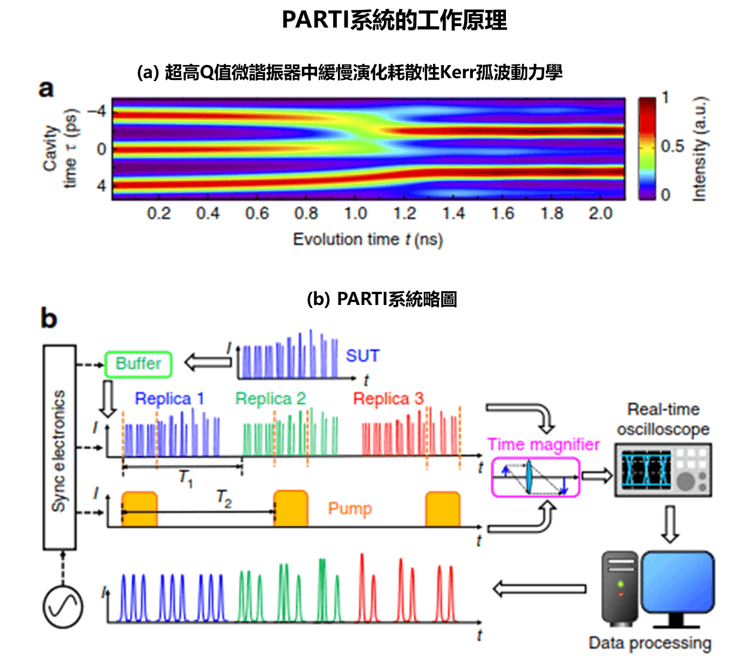
- This cutting-edge blood test for AD leverages world-leading protein detection technology to simultaneously measure the levels of 21 AD-associated blood protein biomarkers involved in multiple crucial biological processes. Unlike traditional blood assays that typically analyze a single biomarker, this multidimensional approach captures a more comprehensive evaluation of the disease status of individuals. This enables earlier and more accurate detection of MCI and AD, as well as precise indication of brain pathology progression.
- Utilizing proprietary machine learning algorithms, this blood test calculates AD risk scores based on the level changes of the 21 blood biomarkers.
- Validated and optimized through a study involving over 1,200 human plasma samples from diverse ethnic groups, this AD risk scoring system demonstrates high accuracy in assessing an individual’s disease status.
- Early and accurate detection of AD and MCI: This blood biomarker test provides a highly accurate, non-invasive, and cost-effective tool for the early detection and monitoring of AD and MCI, with accuracy rates exceeding 96% and 87% respectively. This test can help identify suitable patients for drug treatments and clinical trial studies. Currently, clinical diagnosis of AD mainly relies on cognitive assessment, which can be subjective, and brain imaging or cerebrospinal fluid assessment, which are expensive or invasive, and not widely accessible.
- Comprehensive analysis of AD status: The unique capability of this 21-protein blood biomarker assay is its ability to simultaneously capture the activities of multiple AD-related biological processes in individuals. This reveals dysregulations, such as inflammation and vascular functions, as AD progresses. It enables a more comprehensive and multidimensional evaluation of disease status, providing critical insights for patient stratification and the development of personalized treatment.
- Identification of disease-causing factors for therapeutic development: This blood biomarker study can help identify disease-causing factors of AD, which can serve as novel targets for developing drugs and therapeutic strategies for AD.
- Prize of the Chinese Delegation for Invention and Innovation, International Exhibition of Inventions Geneva (2023)
- Gold Medal with Congratulations of the Jury, International Exhibition of Inventions Geneva (2023)
- Early and accurate detection of AD and MCI, facilitating the screening of suitable individuals for targeted drug treatments in clinical settings
- Close monitoring of disease progression and drug responses, providing critical insights for clinicians to optimize treatment plans
- Multidimensional and comprehensive analysis of an individual’s AD status, facilitating the development of personalized treatments
Hong Kong Center for Neurodegenerative Diseases (HKCeND) was established under the government-funded InnoHK initiative. HKCeND harnesses the power of science to make a translational impact in the development of innovative diagnostic tools and therapeutic strategies for neurodegenerative diseases.
Building on the pioneering work of HKUST's State Key Laboratory of Nervous System Disorders led by neuroscientist Prof. Nancy Ip, HKCeND has fostered cross-institutional R&D collaboration with University College London and Stanford University.
This interdisciplinary collaboration is bringing together prominent scientists in the areas of neuroscience, stem cell biology and artificial intelligence to advance cutting-edge research and deliver promising breakthroughs that improve and transform the live of people worldwide.




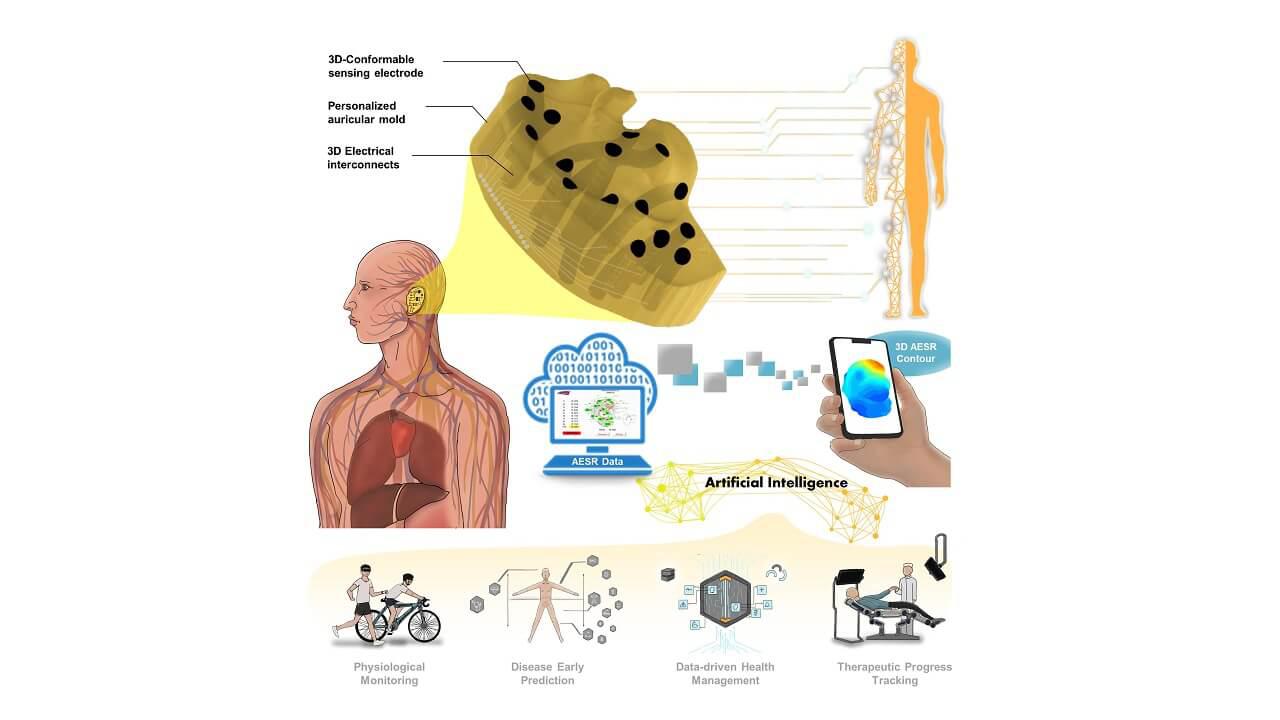



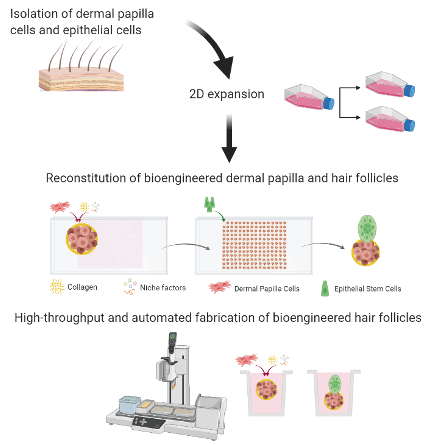




















![Long COVID Test: [Non-Invasive Stool Test to detect distinct gut microbiome profile associated with “Long COVID”]](/uploads/image/202304/6802a81a4ec6afc2ac97f3912ec9a7f4.jpg)