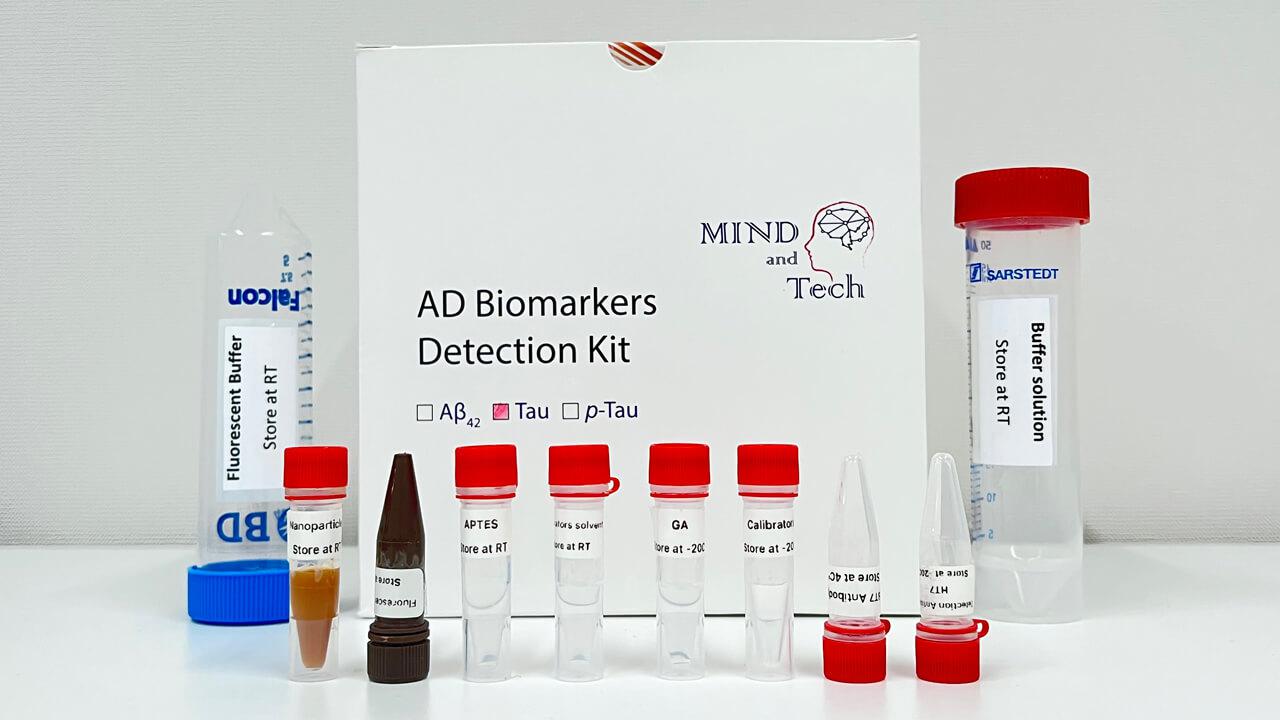
Blood-based Detection Kit for Early Diagnosis of Alzheimer’s Disease

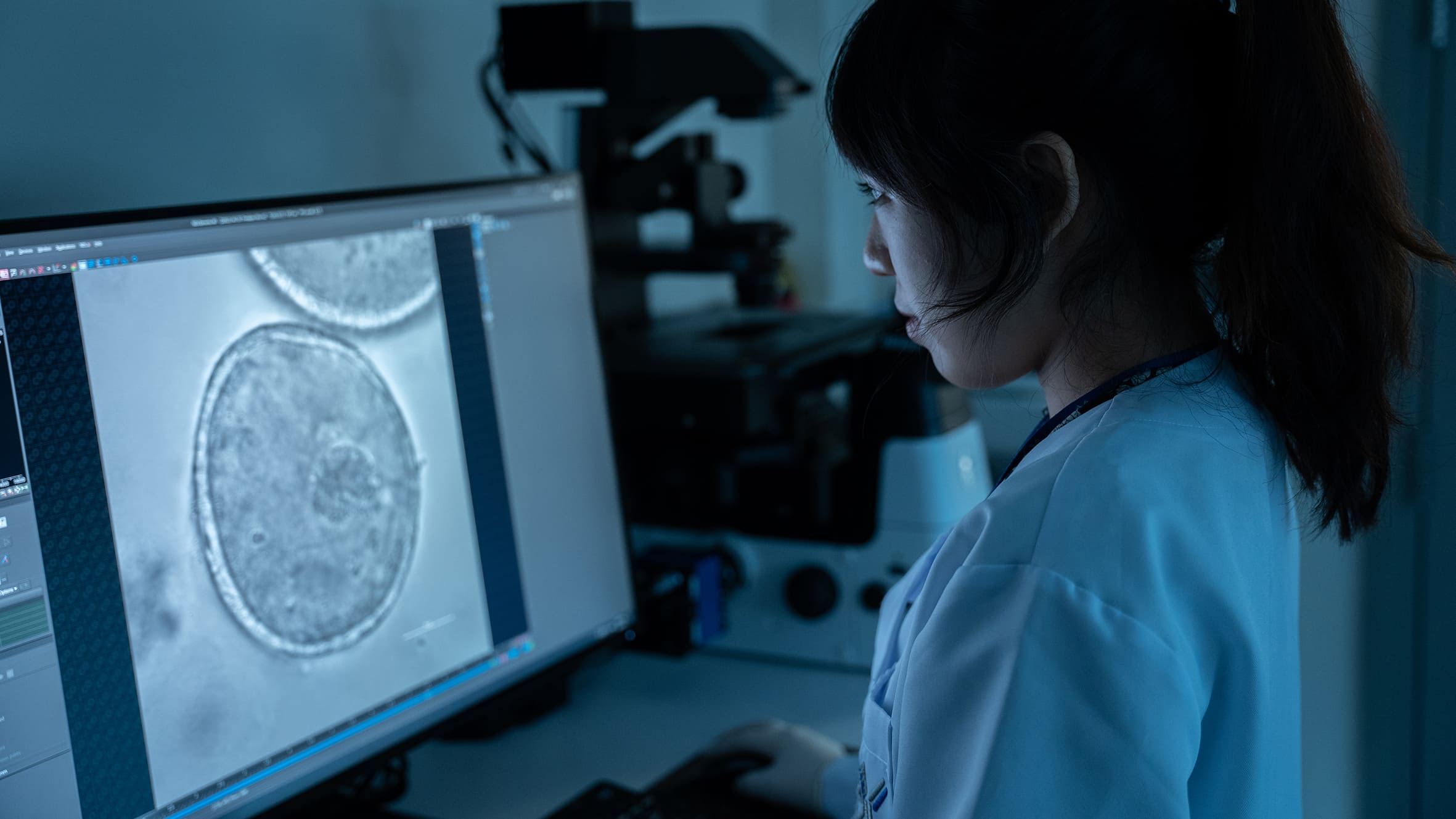
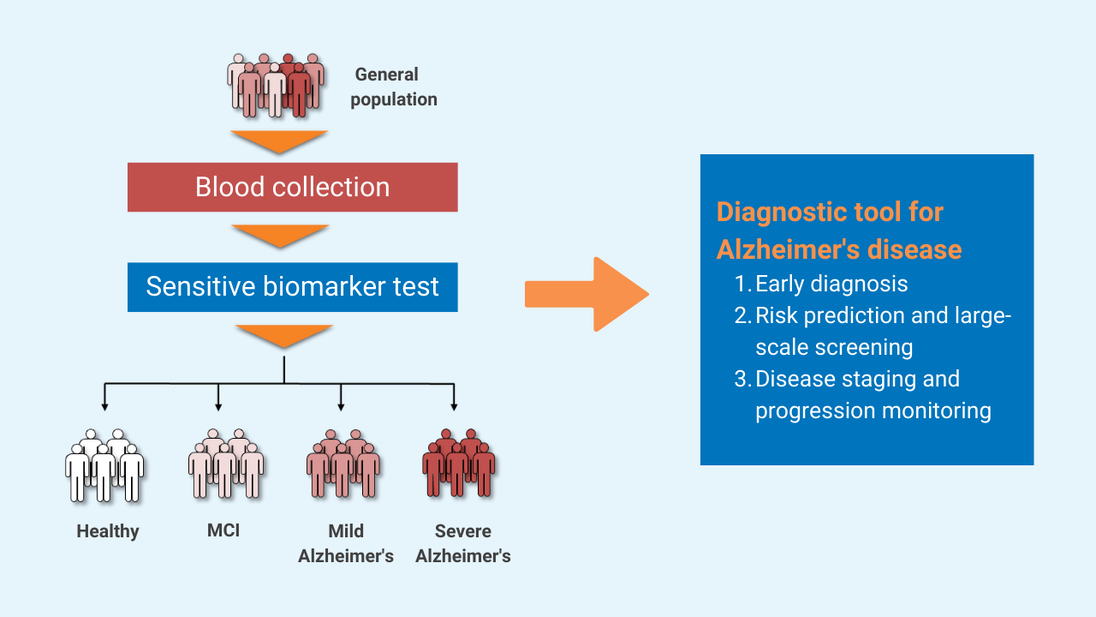
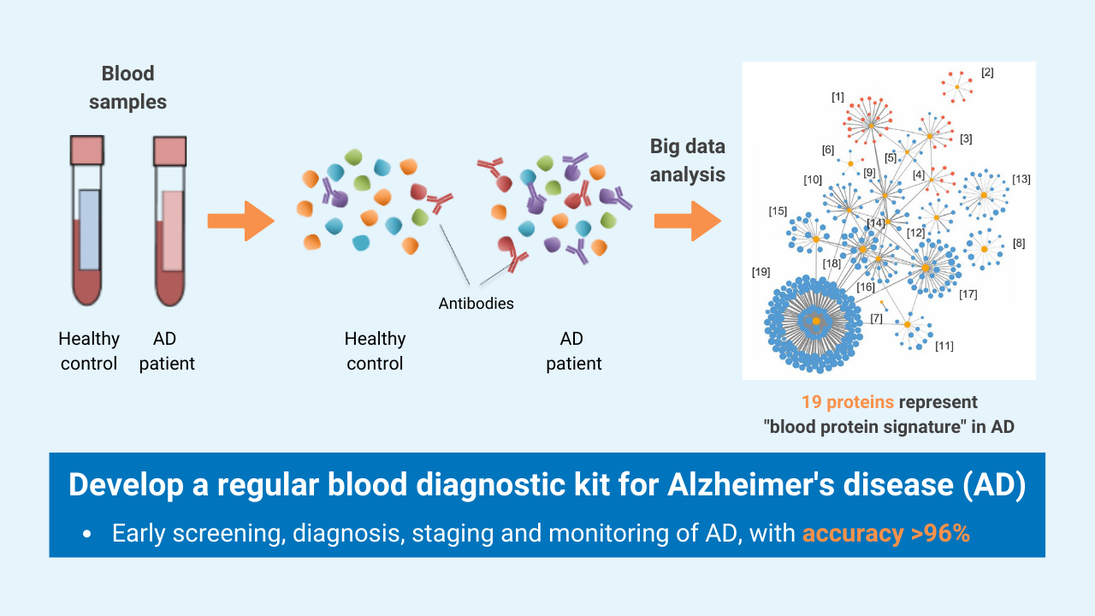
The simple, non-invasive, and accurate diagnostic solution for Alzheimer’s disease (AD) can distinguish patients with AD from healthy people and evaluate disease status from a single drop of blood, with more than 96% accuracy. This cutting-edge technology can be applied towards developing a clinical tool for population-scale screening, early diagnosis, staging and monitoring of the disease.
Alzheimer’s disease is the most common cause of dementia, affecting more than 50 million people globally. Detecting and treating AD remains a significant challenge. This simple and robust blood test for early AD diagnosis and progression monitoring, can address unmet medical need.
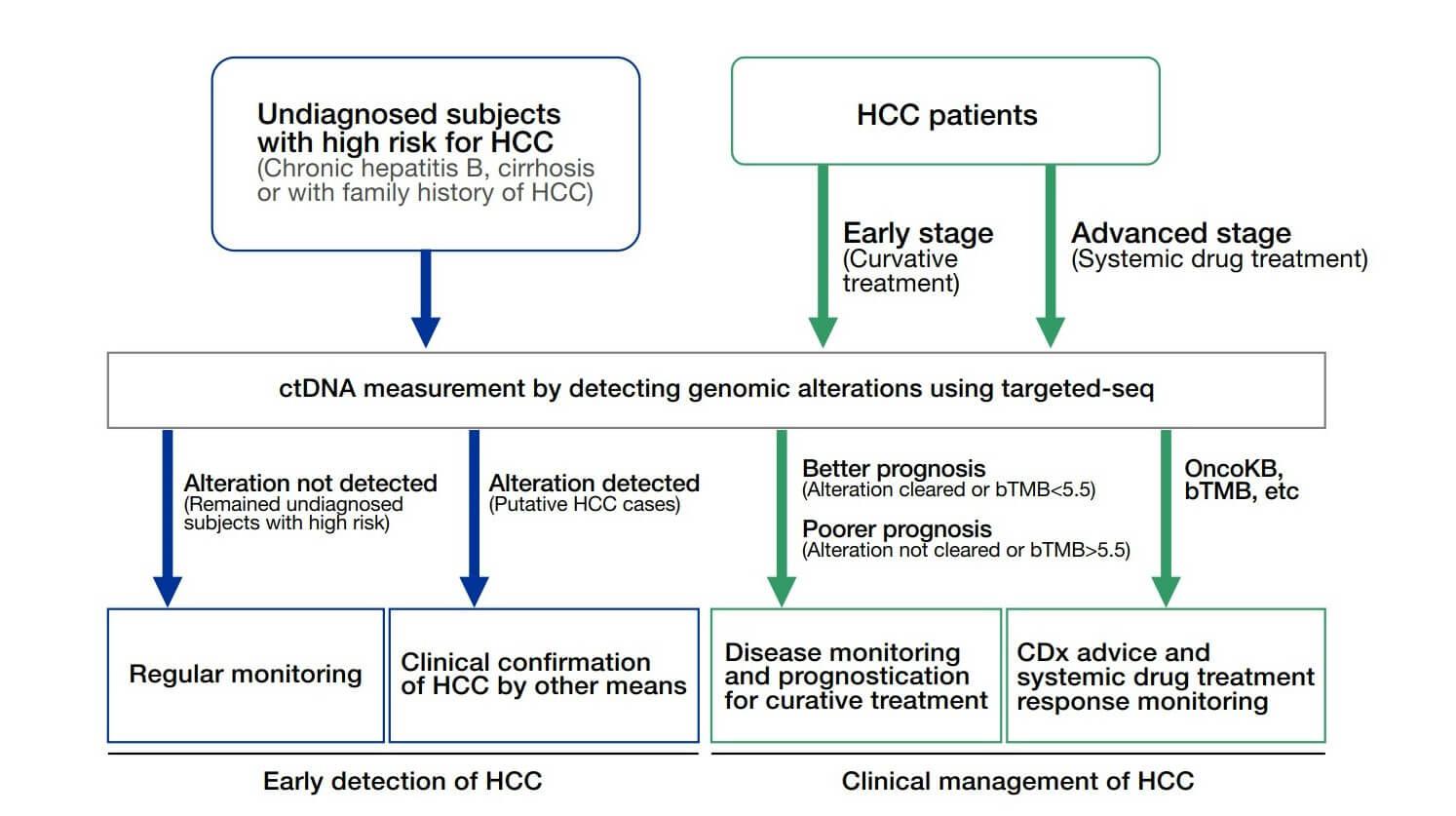
- With the advancement of ultrasensitive and high-throughput blood-based protein measurement technology (i.e. proximity extension assay), a simple and reliable blood test has been developed for AD diagnosis, by detecting a customized panel of novel plasma protein biomarkers. The test can detect AD 5-10 years before clinical symptoms manifest.
- Based on a proprietary self-developed algorithm, this system can differentiate among the early, intermediate, and late stages of AD, and can also be used to monitor the progression of the disease over time.
- Biological changes happen in the brains of AD patients at least 10 to 20 years prior to the appearance of clinical symptoms. This blood test can detect and diagnose AD, when it manifests as mild cognitive impairment or early dementia. It will enable timely intervention/ management of AD.
- This innovative technology detects the levels of 19 AD-associated blood proteins, which can comprehensively capture the status and activities of different body systems, such as neural, immune and metabolic systems, while the existing AD blood tests only detect single protein, which cannot achieve comprehensive, accurate or early detection and monitoring of AD.
- It is a simple and non-invasive solution that can diagnose AD at an early stage. Currently, brain imaging and lumbar puncture are the two most common medical procedures to detect changes in the brain caused by AD, but they are expensive, invasive, and frequently unavailable in particular regions of many countries.
- Prize of the Chinese Delegation for Invention and Innovation, International Exhibition of Inventions Geneva (2023)
- Gold Medal with Congratulations of the Jury, International Exhibition of Inventions Geneva (2023)
- Early diagnosis of Alzheimer’s disease
- Large-scale screening for Alzheimer's disease
- Disease staging and progression monitoring for Alzheimer's disease, which can facilitate personalized intervention
Patent
- Protein Markers for Assessing Alzheimer’s disease (PCT/CN2021/093274)
Hong Kong Center for Neurodegenerative Diseases (HKCeND) was established under the government-funded InnoHK initiative. HKCeND harnesses the power of science to make a translational impact in the development of innovative diagnostic tools and therapeutic strategies for neurodegenerative diseases.
Building on the pioneering work of HKUST's State Key Laboratory of Nervous System Disorders led by neuroscientist Prof. Nancy Ip, HKCeND has fostered cross-institutional R&D collaboration with University College London and Stanford University.
This interdisciplinary collaboration is bringing together prominent scientists in the areas of neuroscience, stem cell biology and artificial intelligence to advance cutting-edge research and deliver promising breakthroughs that improve and transform the live of people worldwide.





















![Long COVID Test: [Non-Invasive Stool Test to detect distinct gut microbiome profile associated with “Long COVID”]](/uploads/image/202304/6802a81a4ec6afc2ac97f3912ec9a7f4.jpg)